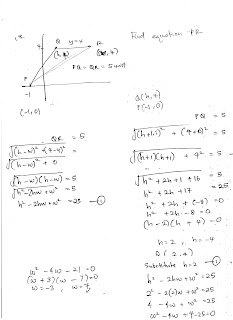
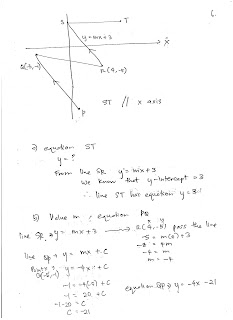
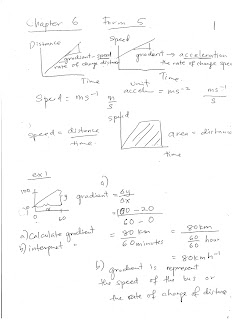
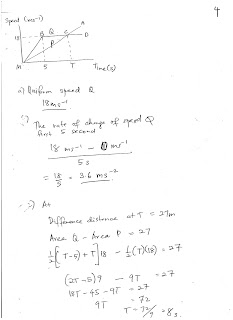
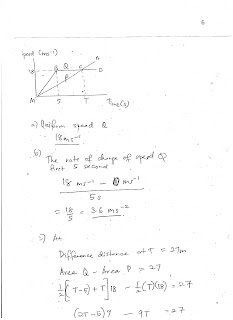
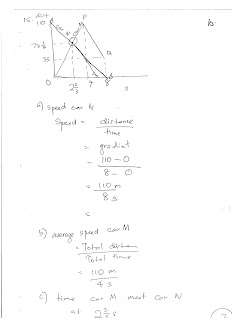
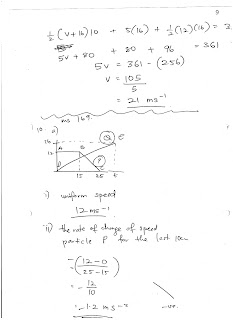
Saturday, August 5, 2017
Wednesday, August 2, 2017
Electrolisis and Voltaic Cell
Electrochemical Cells
An extremely important class of oxidation and reduction reactions are used to provide useful electrical energy in batteries. A simple electrochemical cell can be made from copper and zinc metals with solutions of their sulfates. In the process of the reaction, electrons can be transferred from the zinc to the copper through an electrically conducting path as a useful electric current.
An electrochemical cell can be created by placing metallic electrodes into an electrolyte where a chemical reaction either uses or generates an electric current.
Electrochemical cells which generate an electric current are called voltaic cells or galvanic cells, and common batteries consist of one or more such cells. In other electrochemical cells an externally supplied electric current is used to drive a chemical reaction which would not occur spontaneously. Such cells are called electrolytic cells.
Electrochemical cells which generate an electric current are called voltaic cells or galvanic cells, and common batteries consist of one or more such cells. In other electrochemical cells an externally supplied electric current is used to drive a chemical reaction which would not occur spontaneously. Such cells are called electrolytic cells.
Voltaic Cells
An electrochemical cell which causes external electric current flow can be created using any two different metals since metals differ in their tendency to lose electrons. Zinc more readily loses electrons than copper, so placing zinc and copper metal in solutions of their salts can cause electrons to flow through an external wire which leads from the zinc to the copper.
| Add annotation to illustration |
As a zinc atom provides the electrons, it becomes a positive ion and goes into aqueous solution, decreasing the mass of the zinc electrode. On the copper side, the two electrons received allow it to convert a copper ion from solution into an uncharged copper atom which deposits on the copper electrode, increasing its mass. The two reactions are typically written
The letters in parentheses are just reminders that the zinc goes from a solid (s) into a water solution (aq) and vice versa for the copper. It is typical in the language of electrochemistry to refer to these two processes as "half-reactions" which occur at the two electrodes.
|
|
In order for the voltaic cell to continue to produce an external electric current, there must be a movement of the sulfate ions in solution from the right to the left to balance the electron flow in the external circuit. The metal ions themselves must be prevented from moving between the electrodes, so some kind of porous membrane or other mechanism must provide for the selective movement of the negative ions in the electrolyte from the right to the left.
Energy is required to force the electrons to move from the zinc to the copper electrode, and the amount of energy per unit charge available from the voltaic cell is called the electromotive force (emf) of the cell.
Energy per unit charge is expressed in volts (1 volt = 1 joule/coulomb).
Energy per unit charge is expressed in volts (1 volt = 1 joule/coulomb).
Clearly, to get energy from the cell, you must get more energy released from the oxidation of the zinc than it takes to reduce the copper. The cell can yield a finite amount of energy from this process, the process being limited by the amount of material available either in the electrolyte or in the metal electrodes.
For example, if there were one mole of the sulfate ions SO42- on the copper side, then the process is limited to transferring two moles of electrons through the external circuit. The amount of electric charge contained in a mole of electrons is called the Faraday constant, and is equal to Avogadro's number times the electron charge:
For example, if there were one mole of the sulfate ions SO42- on the copper side, then the process is limited to transferring two moles of electrons through the external circuit. The amount of electric charge contained in a mole of electrons is called the Faraday constant, and is equal to Avogadro's number times the electron charge:
The energy yield from a voltaic cell is given by the cell voltage times the number of moles of electrons transferred times the Faraday constant.
The cell emf Ecell may be predicted from the standard electrode potentials for the two metals. For the zinc/copper cell under the standard conditions, the calculated cell potential is 1.1 volts.
Tuesday, August 1, 2017
Senses in sentences
When talking or writing, it is possible to make use of our five senses. It is also possible to exclude the senses from the what is said, leading to a more objective way of speaking.
Sensory language references and stimulates the senses, thus:
|
The party is frequently described as very colorful. "And already the halls and salons and verandas are gaudy with primary colors.the orchestra is playing yellow cocktail music.the seachange of faces and voices and color."
2. Morning came and the people arrived.
The cool morning sun cast long fingers of shadow and light across the green field as our visitors tramped across rough and the dewy grass.
Objective language seeks to engage the logical mind, but not the senses, which are considered too emotional. It is thus common in such as legal, scientific and business writing
|
The old grandfather and his little grandson
The Old Grandfather and
His Little Grandson
Leo Tolstoy
The grandfather had become very old. His legs would not carry him, his eyes could not see, his ears could not hear, and he was toothless. When he ate, bits of food sometimes dropped out of his mouth. His son and his son's wife no longer allowed him to eat with them at the table. He had to eat his meals in the corner near the stove.One day they gave him his food in a bowl. He tried to move the bowl closer; it fell to the floor and broke. His daughter-in-law scolded him. She told him that he spoiled everything in the house and broke their dishes, and she said that from now on he would get his food in a wooden dish. The old man sighed and said nothing.
A few days later, the old man's son and his wife were sitting in their hut, resting and watching their little boy playing on the floor. They saw him putting together something out of small pieces of wood. His father asked him, "What are you making, Misha?"
The little grandson said, "I'm making a wooden bucket. When you and Mamma get old, I'll feed you out of this wooden dish."
The young peasant and his wife looked at each other, and tears filled their eyes. They were ashamed because they had treated the old grandfather so meanly, and from that day they again let the old man eat with them at the table and took better care of him.
Tell vs Show Sentences
Add sight
She smelled of wet cigarettes and bacon. As they slowly climbed the long, steep staircase, the only sound was his grandmothers' labored breathing and the mournful creak of the wooden stairs.
....................................................................................................................................................
tell vs show
1. Tell sentence
"Mrs Jones love gardening."
Writing which "shows" generally incorporates vivid descriptive detail in order to help the reader evaluate evidence in order to make the appropriate judgments.
Show sentences
From the moment she woke up in the morning, Mrs. Jones smiled at the thought of her garden. Most mornings, she'd scald her mouth trying to gulp down her coffee so that she could get outside while the ground was still damp from the morning dew. Once she knelt down in the soil, she lost track of all time and all concerns of her body. She would work well into the evening, barely noticing when the nails of her left hand would break to the point of bleeding. Mrs. Jones' Jones jeans were always stained on the knees with thick mud, and her arms were always bruised and scraped. But she didn't mind a bit.
2. Tell sentence
Dennis Rodman is a poor role model.
Show sentences
Dennis Rodman continues to break the rules of the NBA. He is rude to officials, excessively violent on and off the court, and has publicly claimed he holds no remorse for his actions.
She smelled of wet cigarettes and bacon. As they slowly climbed the long, steep staircase, the only sound was his grandmothers' labored breathing and the mournful creak of the wooden stairs.
....................................................................................................................................................
tell vs show
1. Tell sentence
"Mrs Jones love gardening."
Writing which "shows" generally incorporates vivid descriptive detail in order to help the reader evaluate evidence in order to make the appropriate judgments.
Show sentences
From the moment she woke up in the morning, Mrs. Jones smiled at the thought of her garden. Most mornings, she'd scald her mouth trying to gulp down her coffee so that she could get outside while the ground was still damp from the morning dew. Once she knelt down in the soil, she lost track of all time and all concerns of her body. She would work well into the evening, barely noticing when the nails of her left hand would break to the point of bleeding. Mrs. Jones' Jones jeans were always stained on the knees with thick mud, and her arms were always bruised and scraped. But she didn't mind a bit.
2. Tell sentence
Dennis Rodman is a poor role model.
Show sentences
Dennis Rodman continues to break the rules of the NBA. He is rude to officials, excessively violent on and off the court, and has publicly claimed he holds no remorse for his actions.
vivid adjectives to develop sensory description
Descriptive writing provides literary texture to a story. Texture shows rather than tells. A writer
shows the reader through the senses of sight, hearing, smell, taste, and touch, as well as through
emotional feelings.
Descriptive details enable the reader to visualize elements in the story.
Vivid adjectives and active verbs help the writer to develop specific sensory descriptions.
For example:
The woman on the beach watched the sun set over the ocean. TELLS
Shades of neon illuminated the edges of clouds, backlit by the sizzling sun that slipped beneath a cerulean sea. SHOWS
#Notice that sentences that TELL tend to be direct. They are objective. Sentences that TELL record verifiable facts as a scientist or journalist might.
Sentences that SHOW are subjective; they may be influenced in part by the writer’s personal experiences. Sentences that SHOW create mental images, and elicit emotional response.
Descriptive details enable the reader to visualize elements in the story.
Vivid adjectives and active verbs help the writer to develop specific sensory descriptions.
For example:
The woman on the beach watched the sun set over the ocean. TELLS
Shades of neon illuminated the edges of clouds, backlit by the sizzling sun that slipped beneath a cerulean sea. SHOWS
#Notice that sentences that TELL tend to be direct. They are objective. Sentences that TELL record verifiable facts as a scientist or journalist might.
Sentences that SHOW are subjective; they may be influenced in part by the writer’s personal experiences. Sentences that SHOW create mental images, and elicit emotional response.
How to change weak verbs to strong verbs
TO REWRITE SENTENCES USING STRONG VERBS:
- Underline any use of Be, Is, Are, Was, Been, Being, Were, Has, Have, Having, Had.John is the manager of the produce department.
- Look for a noun or adjective that you can convert to a strong verb.John is the manager of the produce department. ("manager," noun -- predicate nominative)
- Rewrite the sentence using that strong verb.John manages the produce department.
Always use good grammar in English when you write, but do not neglect using strong verbs for strong writing and a more robust writing style.
Subscribe to:
Posts (Atom)